Over time, vaccine research has evolved significantly, which is now being driven by several innovative technologies, including those involving the use of recombinant deoxyribonucleic acid (DNA) and nucleic acids. Further, with the discovery of more disease targets, players engaged in vaccine development have shifted their focus towards the development of vaccines that target a myriad of disease indications other than infectious diseases. In order to mitigate the aforementioned challenges, pharma and biotech companies are gradually adopting viral vaccine cell culture media for vaccines manufacturing.
A vaccine is a biological substance that is used to stimulate the host’s immune system against a specific disease. It is worth highlighting those vaccines are also referred to as immunizers, due to their ability to take advantage of the host’s natural immune system to prevent illnesses. Generally, the vaccines contain a weakened or an inactivated (killed) form of a virus / a small (in virulent) part of the virus, which is called the antigen. Vaccines help the immune system recognize the antigen as a foreign entity or invading germ, thereby, resulting in the production of antibodies. This process aids the immune system in generating a memory against the specific pathogen so that if the host is attacked by the same pathogen in the future, the body can generate an illicit response.
To request a sample copy / brochure of this report, please visit link
https://www.rootsanalysis.com/reports/viral-vaccine-cell-culture-media-market/request-sample.html
Viral vaccines were first produced in the early 1940s, using embryonated chicken eggs to replicate a broad variety of viruses. Over 30 human vaccines, manufactured using this technique, have been licensed; however, the production capacity associated with this method was limited due to the unavailability of fertilized eggs. Further, in 1950s, an alternative technology based on animal cell culture, which used primary cells as substrate, was developed. Then, in the late 1960s, continuous cell lines were recognized as suitable hosts for human vaccine production, however, the first production process using this technique was established in 1977. One of the key objectives of this report was to evaluate the current opportunity and the future potential associated with the viral vaccine cell culture media market, over the coming decade. We have developed an informed estimate on the likely evolution of the market in the short to mid-term and long term, for the period 2022-2035.
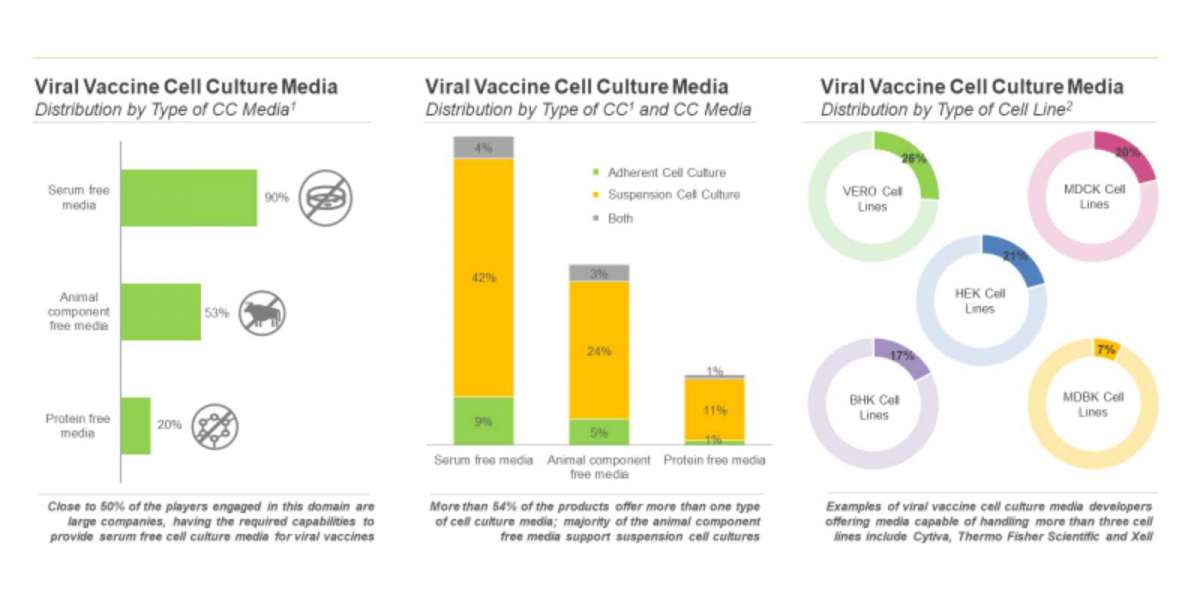
Cell cultures can be broadly classified into two categories, namely adherent and suspension cell cultures.
- Adherent Cell Culture: As the name suggests, these cells must be attached to a surface to grow. These cells can be cultured in flasks, roller bottles and other cell culture vessels capable of handling anchorage-dependent cell types. As most of the tissue-derived cells in the body require an extracellular matrix for growth and proliferation, this method provides scalable options, such as mimicking a microenvironment. In addition, some adherent platforms provide the benefit of visualization through a microscope.
- Suspension Cell Culture: In this process, cells or cluster of cells are allowed to function and multiply in an agitated growth medium. Suspension cells can be cultured in small-scale vessels, such as spinner flasks or large-scale vessels (single-use bioreactors). These cells offer a vast range of scalability, thereby, making it a preferred option for manufacturers in order to increase their operational efficacy. In fact, the nutrients can be continually adjusted in these cell cultures, depending on their usage and need of the cells. However, suspension cell culture lacks the benefit of direct visualization. In addition, the shear force and stress exerted due to the continuous agitation of the media in suspension culture are detrimental to many cell types, especially anchorage-dependent primary and stem cells.
For additional details, please visit link https://www.rootsanalysis.com/reports/viral-vaccine-cell-culture-media-market.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
- Targeted protein degradation market, 2022-2035
- Cell Therapy Manufacturing Market, 2021-2030
- Single-Use Upstream Bioprocessing Technology / Equipment Market, 2022-2035
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at info@rootsanalysis.com
Contact:
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091
Facebook - https://www.facebook.com/RootsAnalysis
LinkedIn - https://www.linkedin.com/company/roots-analysis/mycompany/
Twitter - https://twitter.com/RootsAnalysis
Medium - https://medium.com/@RootsAnalysis
Pinterest - https://in.pinterest.com/RootsanalysisPin/_saved/